Abstract
Introduction
Front-line treatment for patients with active chronic lymphocytic leukemia (CLL) often includes an anti-CD20 monoclonal antibody. However, treatment with anti-CD20 therapy has the potential to reactivate latent hepatitis B virus (HBV). Thus, in 2015 an American Society of Clinical Oncology (ASCO) panel advised screening all patients for HBV infection before starting anti-CD20 therapies. Both Hepatitis B surface antigen (HBsAg) and Hepatitis B core antibody (HBcAb) testing are required for proper screening. In addition, the ASCO panel recommended testing for both IgM and IgG isotypes of HBcAb, as patients with remote HBV infection may have undetectable levels of HBcAb IgM. We evaluated guideline adherence for HBV testing in a large, real-world cohort of patients with CLL.
Methods
This retrospective analysis leveraged data derived from electronic health records (EHRs) in the Flatiron Health database. The database included 7,795 patients with CLL who received at least one order for an antineoplastic treatment between January 1, 2011 and May 31, 2018. We included only patients who received an anti-CD20 therapy (rituximab, ofatumumab, obinutuzumab) and excluded patients whose start of CLL treatment (as captured through chart abstraction) was >30 days before the start of structured EHR data (N=4,288). Using structured EHR data (based on Logical Observation Identifiers Names and Codes [LOINC]), we evaluated the frequency of HBsAg and HBcAb testing, including a separate analysis of HBcAb IgM and IgG testing.
Results
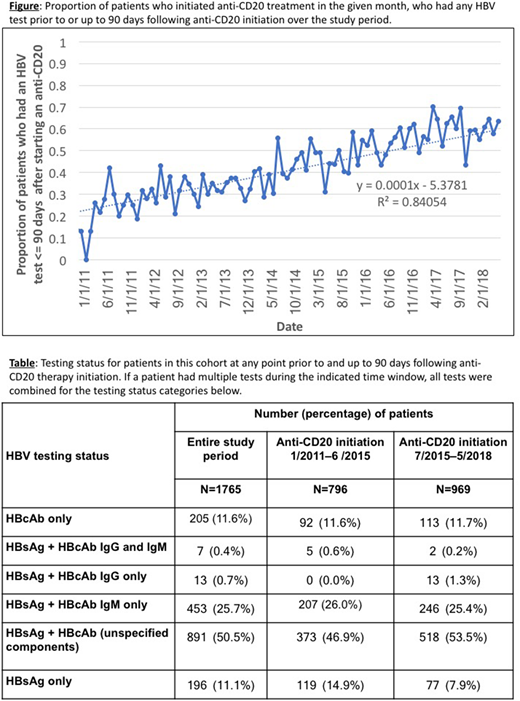
Of 4,288 patients in the cohort treated with anti-CD20, 2,034 (47%) had evidence of HBV testing at any point during their care, and 1,765 (41%) had evidence of HBV testing at any point prior to and up to 90 days following anti-CD20 therapy initiation. Over time, the proportion of HBV infection testing up to 90 days following anti-CD20 treatment initiation increased steadily, from <15% in early 2011, reaching as high as 70% in 2017 (Figure). Following the ASCO guideline publication in 2015, the proportion of patients tested for both HBsAg and HBcAb increased by 33%, while the proportion of HBsAg-only tested patients dropped by 35% (Table). However, even if unspecified HBcAb tests are assumed to represent testing for both IgG and IgM isotypes, in the most recent timeframe nearly half (46%) of the patients who were tested did not receive the requisite screening tests (i.e., for both isotypes). Additionally, nearly 20% received only one test (HBsAg or HBcAb) and another 25% received both tests but the HBcAb test was for the IgM isotype alone (Table).
Discussion
In this real-world cohort of patients with CLL treated with anti-CD20 therapies, overall HBV testing increased throughout the study period, in accordance with ASCO guidelines. Although the most recent data points may not yet capture testing up to 90 days following treatment initiation, and HBV testing information for some patients may not be captured in the EHR, our findings suggest that a sizable proportion of patients are not receiving appropriate screening for HBV. In addition, a significant proportion (25%) of patients were tested for both HBsAg and HBcAb, but the HBcAb test was for the IgM isotype alone, which could result in a false negative test and missed cases of latent HBV. These findings suggest a need for additional quality improvement efforts. Further research is needed to determine demographic and clinical characteristics of tested versus untested patients, and whether testing is associated with reduced risk of liver failure in patients undergoing anti-CD20 therapy.
Hooley:Flatiron Health: Employment. Chen:MedCyclops LLC: Equity Ownership; Nootrobox: Consultancy; Flatiron Health: Employment. Maignan:Flatiron Health: Employment. Carson:Roche: Consultancy; Washington University in St. Louis: Employment; Flatiron Health: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal